Produced by Tianjin Nankai Hecheng, please indicate the source for reprinting
In addition to resin, there are many different methods or procedures for adsorption separation methods.
- Static adsorption is to put multiple cone-shaped flasks containing adsorption resin and solution into the oscillator, oscillate and adsorb at a certain temperature for enough time to make the adsorption reach equilibrium, detect the remaining concentration of the solution, and calculate the adsorption of a certain substance by the resin The amount or adsorption rate. This method is mostly used to determine adsorption isotherms or parallel screening and comparison of multiple resins. Static adsorption is often used in experimental research.
- Dynamic adsorption is column adsorption. Pass the solution into the resin column and perform adsorption at a certain flow rate. After the adsorption is over, rinse with an appropriate amount of pure water to remove the remaining solution, and finally eluted with an appropriate amount of polar organic solvent (commonly used 70% ethanol, methanol or acetone). Whether the adsorption-elution process is suitable is usually judged by adsorption and elution curves. Dynamic adsorption is used both in experiments and in actual production.
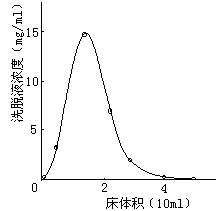
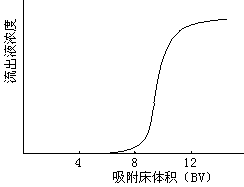
There are many ways of dynamic adsorption separation:
- General adsorption-elution separation is the most used non-polar adsorption resin, which can separate hydrophobic substances from hydrophilic substances. Such as the separation of flavonoid glycosides, saponins, alkaloids, sugars, and inorganic salts.
- Adsorption-step elution separation is used to separate substances with different hydrophobicity. For example, using ordinary non-polar resin to adsorb Ginkgo biloba flavonoid glycosides and eluting step by step with 10%, 20% and 70% ethanol, the content of flavonoid glycosides can be increased from 20% to over 30%. There will be some loss of ester.
- Chromatographic separation (chromatographic separation) is to select the appropriate stationary phase (also known as filler, medium) and mobile phase (eluent), and then flow out of the column. In the elution mode, there are isocratic elution and gradient elution.
Such as the separation of camptothecin and camptothecin:
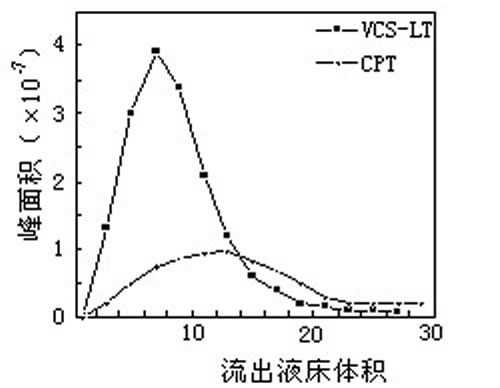
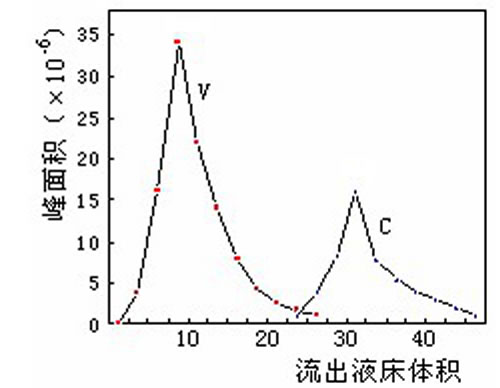
There are many kinds of fillers used for chromatographic separation. From the resin skeleton, there are hydrophobic type (such as polystyrene type, polyacrylate type) and hydrophilic type (such as dextran type, agarose type, cellulose type, shell type). Glycan type, etc.). There are hydrophobic type, ion exchange type, gel filtration type, affinity type and so on from the group. Chromatographic separation can not only separate natural products of small molecules, but also separate biological macromolecular substances such as peptides, proteins and polysaccharides. The key to separation is to select suitable fillers and mobile phases.
